Biostatistics
Biostatistics
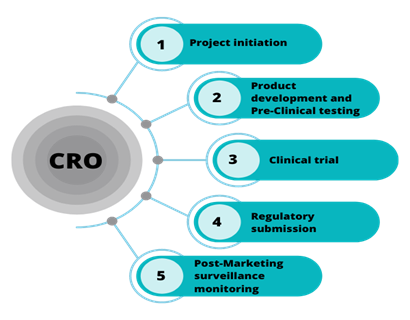
At Weltrix, we understand that biostatistics is the cornerstone of successful clinical research. Our specialized biostatistics services within a Contract Research Organization (CRO) framework are designed to support your clinical development programs from study design through regulatory submission.
Our biostatisticians work closely with your project team to ensure the validity, reliability, and interpretability of clinical trial data throughout all phases of development. We provide expert guidance on study design, protocol development, sample size calculation, randomization, and statistical analysis plans to ensure your trials are scientifically sound and statistically robust.

Key Services in Biostatistics:
Our suite of services covers every stage of the data management lifecycle, including:
Study Design & Protocol Development: Crafting statistically rigorous trial designs and protocols that meet regulatory and scientific standards.
Sample Size Calculation & Randomization: Determining the optimal study size and treatment allocation to reduce bias and improve results reliability.
Statistical Analysis & Reporting: Delivering clear, comprehensive statistical analysis, tables, figures, and reports for clinical study reports and regulatory submissions.
Adaptive Design & Interim Analysis: Implementing innovative trial designs and conducting interim analyses to enhance trial flexibility and decision-making.
Regulatory Support: Providing statistical consultations, meeting representations, and ensuring data compliance with regulatory guidelines such as CDISC standards.
Data Management Collaboration: Working with data management teams to assure integrity and quality of clinical data for analysis.
Our team's expertise spans all clinical trial phases and therapeutic areas, ensuring your project benefits from meticulous biostatistical planning and analysis. Partner with Weltrix to make our biostatistics team an extension of yours, bringing scientific rigor and regulatory confidence to your clinical research.
Contact us today to learn how Weltrix Biostatistics in CRO can support your clinical development needs with precision, quality, and expert insight.
Clinical Programming
Site selection, patient recruitment, investigator oversight.
Regulatory Affairs
Submission planning, with ICH-GCP, DCGI, and FDA norms.
Biostatistics
Includes EDC systems, analysis, reporting, and clinical study documentation.

