Medical Writing
Medical Writing
At Weltrix, we specialize in expert medical writing services tailored for Contract Research Organizations (CROs). Our mission is to provide precise, high-quality, and regulatory-compliant documentation that supports your clinical research and drug development journeys efficiently and effectively.

What We Offer:
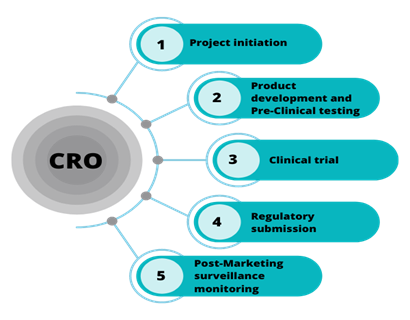
Full Lifecycle Medical Writing Support: From initial clinical study protocols and informed consent forms to comprehensive clinical study reports (CSRs) and regulatory submissions such as IND, NDA, and MAA applications, Weltrix delivers complete documentation aligned with regulatory requirements.
Regulatory Compliance and Clarity: Our medical writers have extensive expertise in creating documents that meet global regulatory standards, including FDA, EMA, and ICH guidelines. We ensure clarity and consistency that enhance the likelihood of regulatory approval.
Therapeutic Area Expertise: Our team brings experience across diverse therapeutic fields, including oncology, rare diseases, neuroscience, pediatrics, and more, allowing us to understand and address your specific scientific and medical communication needs.
Collaborative and Flexible Approach: We work closely with sponsors, project teams, and medical monitors to deliver tailored content on time, adapting our services to fit your project’s changing requirements.
Comprehensive Document Types: Weltrix prepares a broad range of essential clinical documents, including study protocols, investigator brochures, patient narratives, clinical trial applications, safety reports, data presentations, and manuscripts for publication.
Global Reach with Localized Precision: We support multilingual documentation and are well-versed in local regulatory nuances to support clinical trial submissions worldwide.
Why Choose Weltrix?
Our dedicated medical writing team combines scientific rigor with clear, concise communication to streamline your study initiation, execution, and submission processes. By choosing Weltrix, you gain a partner committed to accuracy, quality, and timely delivery, facilitating successful clinical development programs from early-phase studies through to market authorization.
Contact us today to discover how Weltrix Medical Writing Services can enhance the quality and efficiency of your clinical research documentation and support your clinical trial and regulatory submission strategies with excellence.
Clinical Programming
Site selection, patient recruitment, investigator oversight.
Regulatory Affairs
Submission planning, with ICH-GCP, DCGI, and FDA norms.
Biostatistics
Includes EDC systems, analysis, reporting, and clinical study documentation.

