Data Management
Data Management
At Weltrix, we understand that effective data management is critical to the success of clinical research. Our tailored data management services in the CRO industry ensure that your clinical trial data is accurate, reliable, and compliant with regulatory standards—ultimately accelerating your path to meaningful results.

Our Data Management Approach:
We integrate seamlessly with study teams from day one to provide flexible, responsive data management solutions that adapt to your study’s evolving needs.
By emphasizing collaboration and transparency, we prevent costly timeline disruptions and ensure the smooth processing and organization of your clinical data.
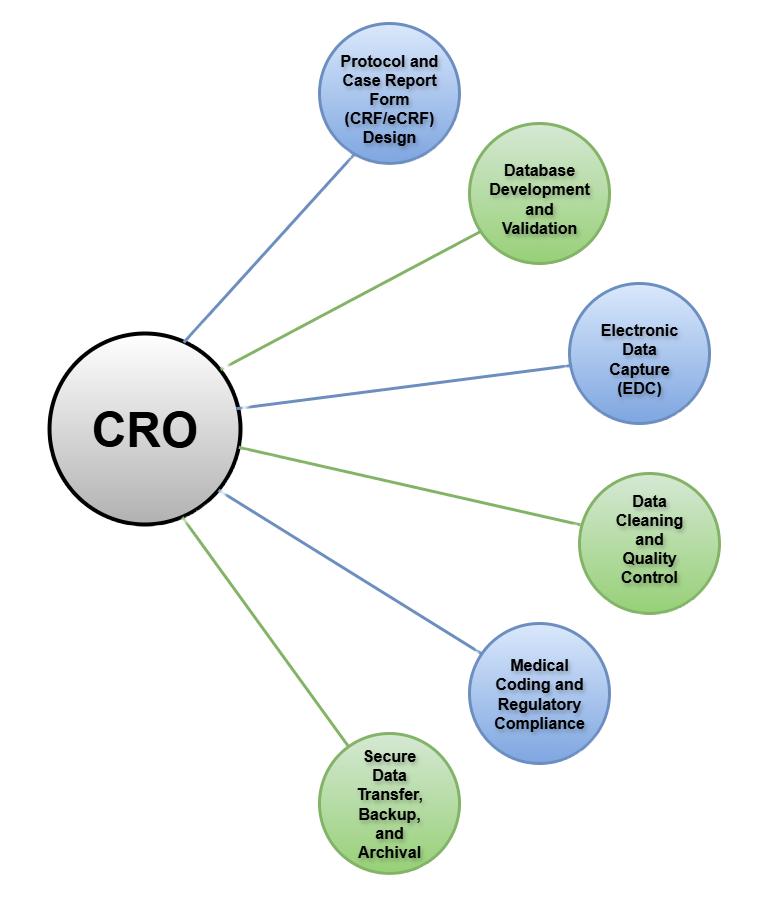
Comprehensive Clinical Data Management Services
Our suite of services covers every stage of the data management lifecycle, including:
Protocol and Case Report Form (CRF/eCRF) Design: We design intuitive CRFs and electronic CRFs that capture all required data elements efficiently and accurately while easing the workload of site staff, monitors, and data managers.
Database Development and Validation: Using industry-standard technology, we build and validate secure, compliant databases tailored to your protocol specifications.
Electronic Data Capture (EDC): Weltrix employs leading EDC platforms to facilitate streamlined data collection, monitoring, and management, ensuring real-time data availability and integrity.
Data Cleaning and Quality Control: Through rigorous data review, edit checks, and quality assessments, we identify and resolve discrepancies to maintain the highest data quality standards.
Medical Coding and Regulatory Compliance: Our expert coders utilize standard and customized dictionaries to code clinical data accurately, supporting regulatory submissions.
Secure Data Transfer, Backup, and Archival: We ensure all clinical data is protected with robust backup and disaster recovery systems, with secure transfer protocols designed to meet client requirements.
Why Choose Weltrix for Your Clinical Data Management?
Flexibility and Expertise: We adjust nimbly to changes and challenges, ensuring timelines are met without compromising data quality. Integration with Study Teams: Data management is not an isolated function; we embed ourselves within your project team to enhance communication and efficiency. Technology-Driven Solutions: Leveraging cloud-based EDC systems and tailored software tools, we optimize data handling and reporting to maximize productivity and minimize error. Strong Focus on Data Quality and Compliance: Every step of our process is designed to meet or exceed benchmarks set by clinical data standards organizations and regulatory agencies.
Clinical Programming
Site selection, patient recruitment, investigator oversight.
Regulatory Affairs
Submission planning, with ICH-GCP, DCGI, and FDA norms.
Biostatistics
Includes EDC systems, analysis, reporting, and clinical study documentation.

